Original story at U of U Health.
The experts agree — the pandemic is not over. Infections are ticking up again, fueled by new variants our immune systems are ill prepared for.
That’s according to a study which found that the antibodies generated in people who were vaccinated and/or recovered from COVID-19 prior to 2022 failed to neutralize the variants circulating today.
The study was led by Shawn Owen, PhD, an assistant professor of pharmaceutics and pharmaceutical chemistry at University of Utah Health and Igor Stagljar, PhD, a professor of biochemistry and molecular genetics, at the University of Toronto’s Temerty Faculty of Medicine.
The journal Nature Communications published their findings.
The researchers say the antibody test they developed to measure immunity in the study’s participants will be a valuable tool for deciding who needs a booster and when, which will help save lives and avoid future lockdowns.
“As the pandemic stretches on, we need ways to determine if people are protected from infection or reinfection,” Owen says. “Our assay can help monitor the level of immunity a person has after they’ve been vaccinated or infected. It can also reveal the level of protection against new variants which can help guide decisions about when to get a booster or if a new vaccine is needed.”
Many antibody tests have been developed over the past two years. But only a few authorized tests are designed to monitor neutralizing antibodies, which coat the viral spike protein so that it can no longer bind its receptor and enter cells.
It’s an important distinction, as only a fraction of all Sars-CoV-2 antibodies generated during infection are neutralizing. And while most vaccines were specifically designed to produce neutralizing antibodies, it’s not clear how much protection they give against variants
“The truth is we don’t yet know how frequent our shots should be to prevent infection,” Stagljar says. “To answer these questions, we need rapid, inexpensive and quantitative tests that specifically measure Sars-CoV-2 neutralizing antibodies, which are the ones that prevent infection.”
To meet this need, the research team developed Neu-SATiN, which stands for Neutralization Serological Assay. The effort was was spearheaded by Sun Jin Kim, a postdoctoral fellow in Owen’s lab and Zhong Yao, a senior research associate in Stagljar’s lab, who are the co-first authors on the paper.
The method is as accurate as, but faster and cheaper than, the current gold standard assay, and it can be quickly adapted for new variants as they emerge, according to the researchers.
“The biggest advantage of Neu-SATiN over other surrogate assays is the modularity,” Kim says. “Each of the Neu-SATiN’s assay components are genetically engineered and recombinantly expressed making them relatively easy to modify and produce. This enables Neu-SATiN to be a truly “mix-and-read” assay.”
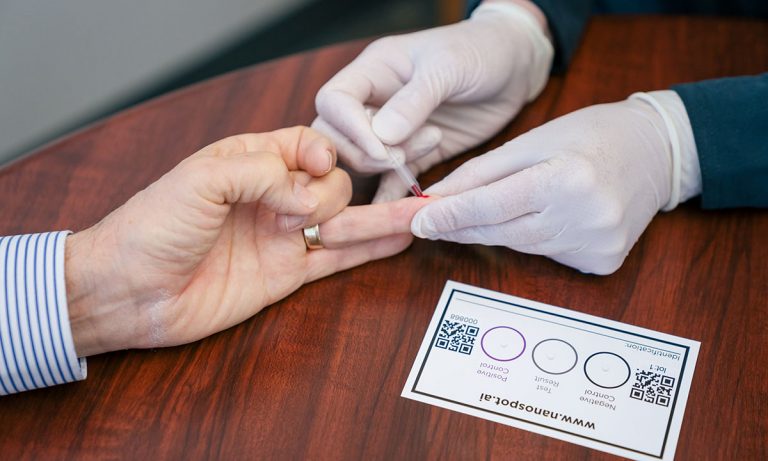
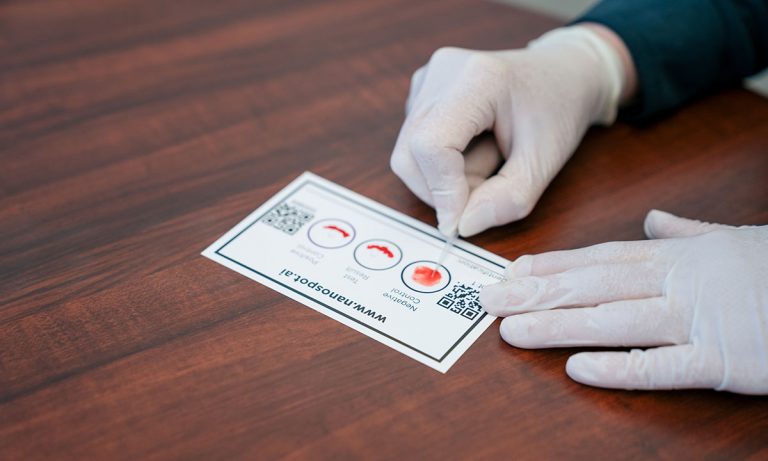
The pin prick test is powered by the fluorescent luciferase protein from a deep-water shrimp. It measures the ability of the viral spike protein to bind the human ACE2 receptor, each of which is attached to a luciferase fragment.
The binding brings the luciferase pieces into proximity so that they reconstitute a full-length protein, which gives off a glow of light that is captured by instrumentation. When patient blood sample is added into the mixture, the neutralizing antibodies will bind the spike protein, preventing it from contacting ACE2. Luciferase remains in pieces, with an accompanying drop in light signal.
The plug and play method can be adapted to different variants within a couple of weeks by engineering variant mutations into the spike protein.
The researchers applied Neu-SATiN to blood samples collected from 63 patients with different histories of COVID-19 infection and vaccination up to November 2021. Their antibody neutralizing capacity was assessed against the original Wuhan strain and the variants, Alpha, Beta, Gamma, Delta and Omicron.
The neutralizing antibodies were found to last about three to four months. At that time, their levels dropped by about 70 per cent irrespective of infection or vaccination status. Hybrid immunity, acquired through both infection and vaccination, produced higher antibody levels at first, but also dropped significantly four months later.
Most worryingly, infection and/or vaccination provided good protection against the previous variants, but not Omicron, or its sub-variants, BA.4 and BA.5.
The researchers stress that vaccines still confer significant protection from severe disease and death. However, that the findings from Owen’s team and others call for vigilance in the coming period, given that the more transmissible BA.4 and BA.5 sub-variants can escape immunity acquired from earlier infections with Omicron. as attested by rising reinfections.
Moving forward, the researchers hope to expand its availability.
“We are working with a few companies to assess the efficacy of their vaccine candidates against Omicron and also negotiating a license with another company to commercialize the assay,” Owen says.
The research was supported with funding from the Office of the Vice President for Research and the 3i Initiative at the University of Utah, and the Toronto COVID-19 Action Fund.